-
Extension point for running JUnit tests in a RCP Application instance?
One thing that has been on my wishlist is to be able to run the unit tests we have for Bioclipse from inside a running Bioclipse instance. That is, we have a Bioclipse Test Suite features on the update site, matching the functional features we have there. Each such test suite would run all JUnit tests we have for that feature. -

JChemPaint update: merging of patches and CDK statistics
With the JChemPaint workshop just passed, there is much work from UU and the EBI to be integrated. Moreover, Rajarshi just merged a lot of fixes from CDK 1.2.x into the master branch, which will be a big rebase too. That said, I need to do this to recalculate source code statistics for the CDK. -
Really free chemistry books
With pleasure I read Analogue or Digital? - Both, Please. Funnily, I just created MP3 (or, preferably Ogg Vorbis, superior but hardly any support by commercial companies, who rather seem to pay license fees) directly from the CD. -
VRMS meme: how much non-free software are you running?
Over at Planet Ubuntu there is a meme running around VRMS (Virtual Richard M. Stallman, brilliant name!) which finds non-free software on your desktop. I uninstalled Sun’s Java6, for which there is the OpenJDK6 alternative. -
Bioclipse, RDF and defeasible reasoning
Well, I have yet to read the paper in detail, but my new student Samuel is going to work for 20 weeks on defeasible reasoning with DrProlog in Bioclipse. -
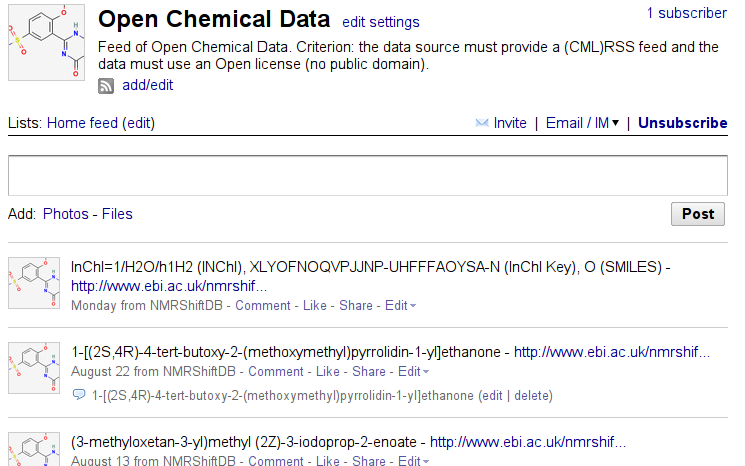
Open Chemical Data #1: NMRShiftDB
As I reported earlier, progress is only possible of you can modify and redistribute. This is why Open Data, Open Source, and Open Standards are so important to us Blue Obelisk members. For data, proper licensing makes these two requirements possible, but more importantly, make those rights explicit. Rich is running the nice Zusammen blog, but most of his entries are not Open Data. Even larger chemistry data repositories can be vague and have seemingly contradicting statements.
